Interventional Care


We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
A little history: In 1818, French Chemist Louis Jacques Thénard, discovered hydrogen peroxide (H2O2), a strong oxidant that has had many uses through history — a bleaching agent for paper and textiles, a rocket propellant and, of particular interest to the healthcare community, a disinfecting agent [1][2]. In 1977, the Environmental Protection Agency (EPA) registered the first hydrogen peroxide pesticide product for disinfecting surfaces[3].
The Chemistry: In acidic solutions, hydrogen peroxide is a powerful oxidizer, stronger than chlorine. Hydrogen peroxide is effective against a wide range of microorganisms, including bacteria, yeasts, fungi, viruses.
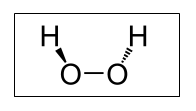
Chemical structure of hydrogen peroxide
With careful formulation considerations, it is also efficacious against spores. It acts as a germicide by producing destructive hydroxyl free radicals that attack a pathogen’s membrane lipids, proteins, DNA and other essential cell components [4][5].
The Benefit over Bleach: One of the key advantages of hydrogen peroxide over bleach (sodium hypochlorite) is that it has a more favorable compatibility profile with most materials commonly used in healthcare environments, particularly with stainless steel.
Alkaline oxidizing salts, such as sodium hypochlorite, are among the most corrosive salts [6]. When a surface disinfected with sodium hypochlorite dries, it leaves behind a residue of sodium salt which can be quite noticeable on certain surfaces. Hydrogen peroxide-based formulas do not leave undesirable residues on surfaces, eliminating additional cleaning steps. Additionally, hydrogen peroxide degrades into environmentally innocuous water and oxygen.
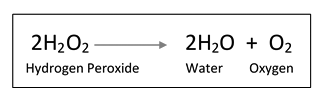
Decomposition of hydrogen peroxide
Hydrogen peroxide degradation is hastened by exposure to light, heat, pH and the presence of metals such as copper, manganese and iron [7]. Therefore, when developing a stable and efficacious hydrogen peroxide-based disinfectant, careful consideration must be given to the selection of formula components, packaging materials and manufacturing equipment and conditions.
Our Solution: PDI’s Sani-HyPerCide™ Germicidal disinfectant:
Powering Through a Pandemic: The Centers for Disease Control (CDC) updated their recommendations for EPA-registered disinfectants to refer to the EPA’s List N: Disinfectants for Use Against SARS-CoV-2 (COVID 19). Sani-HyPerCide Germicidal Disinfectants are included on List N .
Request more information from your PDI Rep and visit the product listing!
*PDI data on file. ASTM D4488-A5. October 11, 2018.