Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
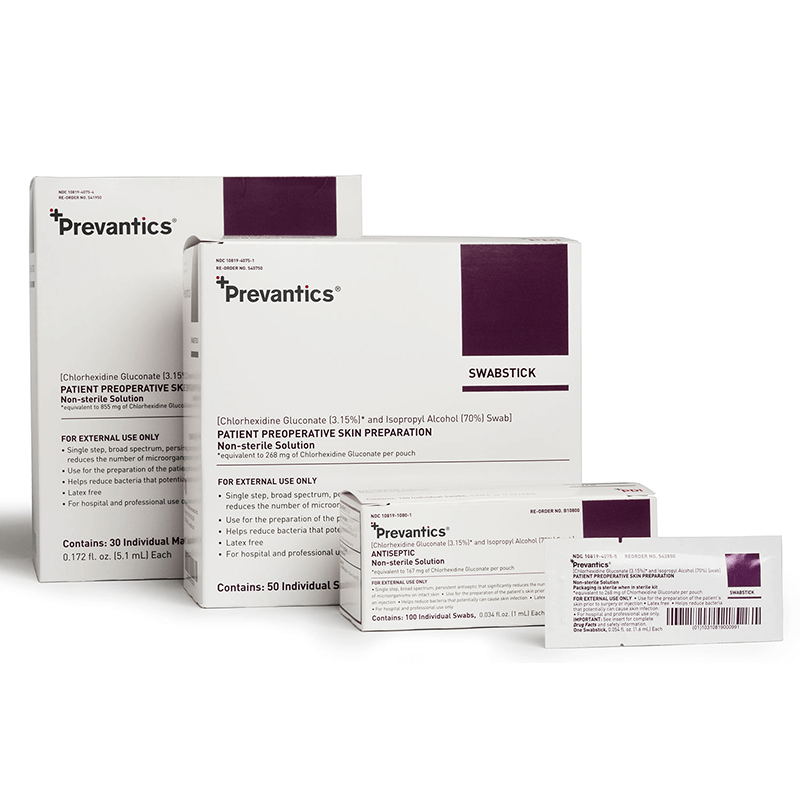


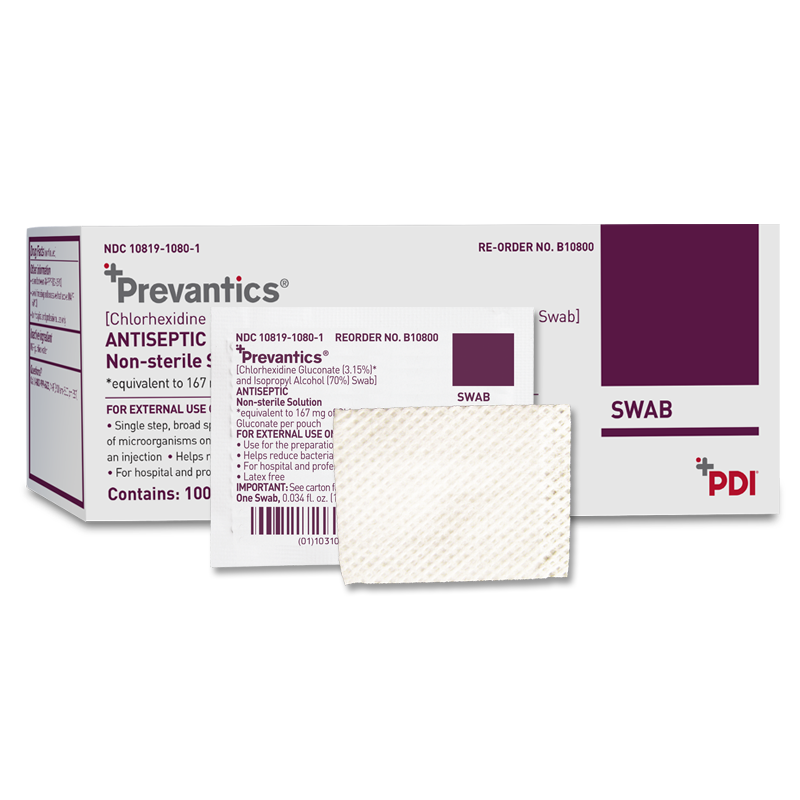


Prevantics Skin Antiseptics are formulated with 3.15% chlorhexidine gluconate (CHG) and 70% isopropyl alcohol (IPA) and help reduce microorganisms that can cause central-line associated bloodstream infections (CLABSIs).
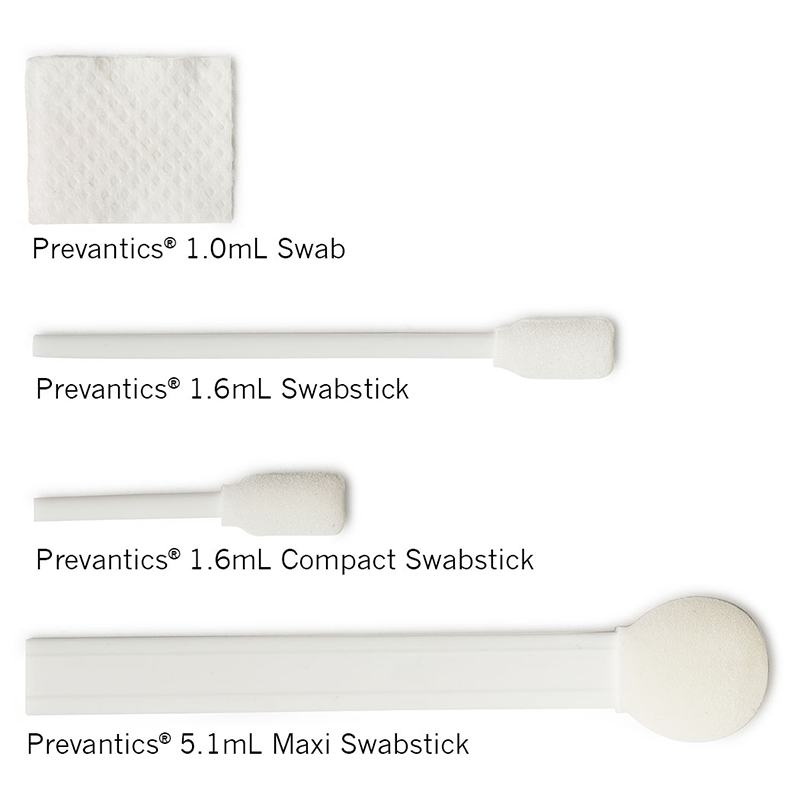
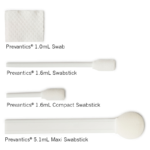
Prevantics skin antiseptics are available in a variety of applicators: Swab, Swabstick, Compact Swabstick, and Maxi Swabstick
SELECT A SIZE
Swab: 15 second scrub/30 second dry time; Swabstick and Maxi Swabstick*: 30 second scrub/30 second dry time
3.15% (w/v) chlorhexidine gluconate (CHG) and 70% (v/v) isopropyl alcohol (IPA)
Broad-spectrum antiseptic that kills a wide range of pathogens including most bacteria, fungi, and yeast.
Nine independent clinical studies demonstrating clinical efficacy of Prevantics products.
Help lower your blood culture contamination rates with a standardized solution: Use Prevantics® antiseptics for skin and blood…
Discover what goes into calculating a HAC score, the science behind the Prevantics 3.15% CHG / 70 % IPA…
This video showcases the 5 different potential sources of Central Line Associated Bloodstream Infections (CLABSIs) and the top…
Discover how facilities across the U.S. have experienced reduced Central Line Associated Bloodstream Infection (CLABSI) rates after implementing…
This video provides an overview of the benefits of Prevantics® Device Swab and how it compares to alternative…
Presented at the 2018 Annual Association for Vascular Access Scientific Meeting. Representatives from facilities who implemented the Prevantics® Device…
HAC scores are complicated and can be difficult to understand. This video gives a brief overview of what…
CLABSIs make up 36% of excess cost in US hospitals today. Central line-associated blood stream infections (CLABSIs) are…
This video is a success story for one facility who implemented Prevantics® Device Swab.
PDI offers a broad range of evidence-based, market-leading Interventional Care, Environment of Care, and Patient Care solutions, all…
Do I need to wipe off the Prevantics® antiseptic liquid from the skin after application?
No. Prevantics contains both 3.15% Chlorhexidine Gluconate and 70% Isopropyl Alcohol which should be left on the skin to provide continued antimicrobial activity.
What are considered "dry" sites and "wet" sites?
Dry sites are areas where there are no skin folds, such as the abdomen, back and forearm, and no evidence of moisture caused by diaphoresis. Wet sites are areas with skin folds, such as the groin area, under arms, and under breasts, as well as areas that may be moist due to diaphoresis.
What is the difference between an occlusive and semi-occlusive dressing?
An occlusive dressing is a non-permeable dressing, which means that no air or moisture can penetrate in or out. A semi-occlusive (semi-permeable, transparent) dressing allows the wound to “breathe” (air can penetrate in and out) but at the same time, protects the wound from outside liquids. Commonly used dressings in vascular access are semi-occlusive, transparent dressings.
Why are Prevantics® Skin Antiseptics applied using back and forth strokes?
The back and forth strokes provide friction which allows for deeper penetration of the antiseptic into the cracks and fissures of the skin in the epidermis.
Some PDI products state "store at room temperature." What is the definition of room temperature?
For our EPA-regulated products, such as Sani-Cloth® and Sani-Prime™ brand products, room temperature is defined as an average temperature of 25◦ C (77◦ F) and within a temperature range of 15◦ C to 30◦ C (59◦ F to 86◦ F). For our FDA-regulated products, such as Prevantics® brand products, “controlled room temperature” indicates a temperature maintained thermostatically that encompasses the usual customary working environment of 20◦ C to 25◦ C (68◦ F to 77◦ F). SOURCE: USP 41-NF 36 General Notices and Requirements (August 1, 2013 First Supplements) Section 10.30.50. “Room Temperature” indicates the temperature prevailing in a working area. Section 10.30.60. Controlled Room Temperature
What is the shelf life for Prevantics® products?
The shelf life for Prevantics® Skin Antiseptics and Prevantics® Device Swab & Strip is 24 months or 2 years.
| SKU Number | Product Size | Dimensions | Packaging | GTIN | Country of Manufacturer |
|---|---|---|---|---|---|
| B10800 | Swab | 1.3125” x 3.1875” | 10/100's | 20310819000011 | USA |
| S40750 | Swabstick | 4.75" x 6.5" | 10/50's | 20310819000028 | USA |
| S41950 | Maxi Swabstick | 6" x 8" | 10/30's | 20310819008413 | USA |
| B11400 | Swab (bulk) | 1.1875” x 3.1875” | 1/3000's | 20310819000868 | USA |
| S32450 | Swabstick (bulk) | 4.75" x 6.5" | 1/500's | 20310819000875 | USA |
| S42850 | Compact Swabstick (bulk) | 4" x 1.75" | 1/500's | 20310819000998 | USA |
| S27350 | Maxi Swabstick (bulk) | 6" x 8" | 1/300's | 20310819000882 | USA |
*for dry sites only.