Interventional Care


We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
Prior to 2020, there had been a significant ongoing and sustained reduction in the rate of central line-associated bloodstream infections in the United States.1 This was followed by a 24% increase in 2020 and additional year-over-year 7% increase in 2021. 2,3
In 2019, the Centers for Disease Control and Prevention (CDC) solicited feedback regarding expanding its surveillance protocols to include hospital onset bacteremia (HOB). 4 A HOB is defined as a first positive blood culture for a noncommensal organism on or after day 3 of hospitalization plus receipt of a new antimicrobial. 5 The scope would include “all bloodstream infections that develop in patients following hospital admission. Although this scope would be wider than Central Line-associated Bloodstream Infection (CLABSI) surveillance, CLABSI surveillance could be incorporated as a subset of HOB surveillance.” 4 The template for this surveillance module has been described in a recent article in Infection Control and Hospital Epidemiology. 5 Further actions towards the adoption of such a measure was presumably stalled with the onset of the pandemic, but as we turn the page to a “new normal” it would not be surprising to see this surveillance measure deployed and potentially ultimately serve as an additional measure for the Centers for Medicare and Medicaid Services (CMS) to use “in its public reporting and payment programs.”. 4 A recent analysis of over 9.2. million admissions across 267 hospitals demonstrated the feasibility of benchmarking facilities with this metric and future adoption as a new CMS reportable measure of healthcare quality seems likely. 5
The rationale for deploying such a metric is readily apparent and arguably logical. This measure seems highly conducive to electronic surveillance, CLABSIs can remain a subset of these infections and the simplification and standardization may theoretically level the playing field between facilities. 5,6 Counter arguments to this proposed measure may include a disconnect between surveillance and clinically relevant definitions. In a recent report, hypothetical HOB scenarios were posed to infectious disease physicians for assessment of preventability. While 44% of the scenarios were indeterminable, 27% were deemed to be preventable while 29% were assessed as not being preventable. 7 It is an imperfect measure in that in an ideal (yet unrealized) world, the majority of HOBs would be preventable and there would be no differentiation between surveillance criteria and clinical diagnosis.
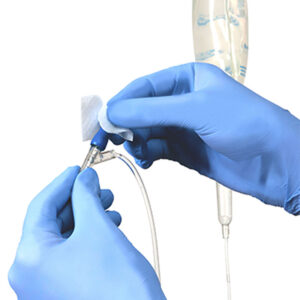
Surveillance for hospital onset bacteremia is expanding beyond the central venous catheter and specific pathogens. We can’t midline our way out of this and it is time to expand our prevention strategies to address all vascular access devices.
All vascular access devices (VAD) bypass the human body’s natural defense of the skin and as such the same risk mitigation strategies for preventing infection (e.g. hand hygiene, skin antisepsis prior to insertion, aseptic non-touch technique, etc) should be deployed. Similarly, needless connectors (NC) of VAD need to be disinfected prior to each access.8 While some recent studies have shown that VAD alternatives to central venous catheters may have a lower risk of infection, that risk is not zero and may come at the price of mechanical complications. 9-12 Although passive disinfection of NC may be a useful adjunct for all VAD, most recommendations favor active, physical scrubbing the hub. 8,13 A recent study showed scrubbing the hub with swabs was more effective than scrubbing with an alcohol based cap and that a majority of staff preferred the shorter scrub and dry time of Prevantics® Device Swab. 14