Interventional Care


We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
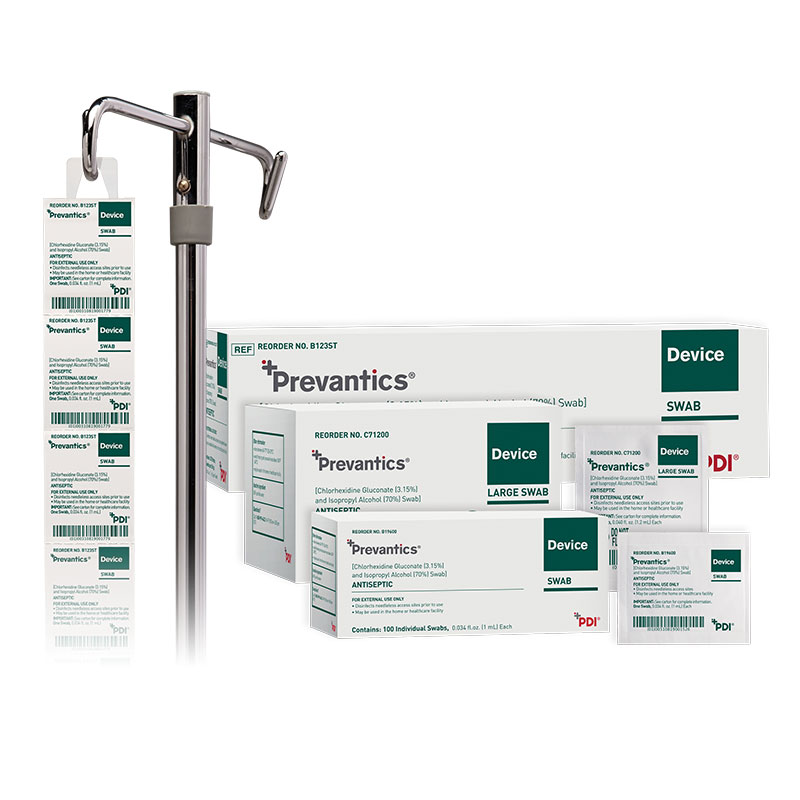

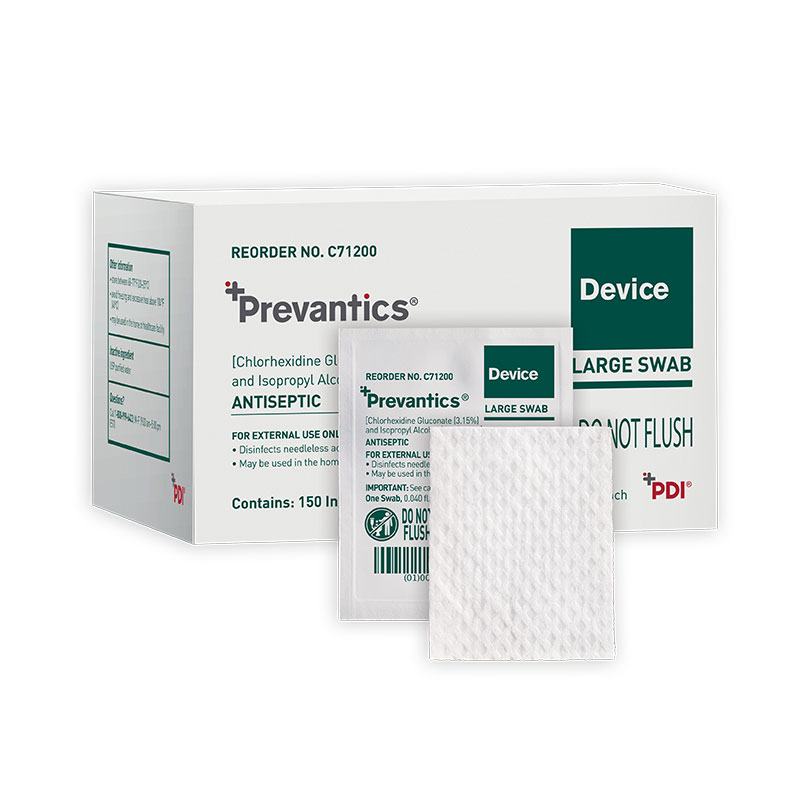






Prevantics Device Swab is formulated with 3.15% chlorhexidine gluconate (CHG) and 70% isopropyl alcohol (IPA). It is conveniently designed to help disinfect needleless access sites and helps improve ‘scrub the hub’ compliance to better protect patients from CLABSIs.
Want to take prevention further? Through our CATALYST Program, customers gain added support in improving clinical metrics and reducing CLABSIs. Plus, with our money-back guarantee, you can be confident in the outcomes. Learn how to become part of the program today.
SELECT A SIZE
5 second scrub time followed by 5 second dry time
3.15% (w/v) chlorhexidine gluconate (CHG) and 70% (v/v) isopropyl alcohol (IPA)
Intuitive, easy-to-use swab format provides friction to remove microorganisms from grooves and threads of connectors
Achieved a >4.0 log10 reduction versus a leading alcohol impregnated cap1 against 7 tested clinically relevant microorganisms known to cause CLABSIs2
Effectively scrub the hub prior to and between each line access, as recommended in the Infusion Nurse Society (INS) Guidelines
Over 15 independent clinical studies demonstrating clinical efficacy of Prevantics products
CDC, INS, SHEA, The Joint Commission National Patient Safety Goals, Infectious Diseases Society of America, APIC, AVA
This video provides an overview of the benefits of Prevantics® Device Swab and how it compares to alternative…
This instructions for use sign contains step-by-step directions on how to properly open the package and disinfect needleless…
“Scrubbing the hub” each time before accessing a line can decrease the chance of CLABSI causing organisms entering…
This video showcases the 5 different potential sources of Central Line Associated Bloodstream Infections (CLABSIs) and the top…
Discover how facilities across the U.S. have experienced reduced Central Line Associated Bloodstream Infection (CLABSI) rates after implementing…
In 2011, The Infusion Nurses Society (INS) clarified the proper steps to disinfect needleless access sites (needleless connectors,…
Discover what goes into calculating a HAC score, the science behind the Prevantics 3.15% CHG / 70 % IPA…
Prevention of Intraluminal Contamination of Intravascular Catheters: Making the Case for Needleless Connector Disinfection with CHG / IPA…
PDI offers a broad range of evidence-based, market-leading Interventional Care, Environment of Care, and Patient Care solutions, all…
How is Prevantics® Device Swab used?
Prevantics Device Swab is designed to disinfect needleless access sites (or to scrub the hub) prior to use as well as in between each line access. Prevantics Device Swab is used to scrub the access site for 5 seconds with drying of the access site for at least 5 seconds.
If I use the Prevantics® Device Swab product, will I still need to use an alcohol impregnated cap?
Not necessarily. If clinicians follow all existing infection prevention and control measures, such as hand hygiene, use of personal protective equipment, use of chlorhexidine-based skin antisepsis, etc. then the use of an alcohol impregnated cap may not be clinically indicated. This would be evaluated by ongoing surveillance of HAI rates.
What evidence-based clinical guidelines support the use of the Prevantics® Device Swab for disinfection of needleless access sites prior to use?
The following evidence-based clinical guidelines support the use of Chlorhexidine Gluconate/Alcohol for disinfection of needleless access sites prior to use:
1. Guidelines for the Prevention of Intravascular Catheter-Related Infections, US Centers for Disease Control and Prevention (CDC)
2. Standards of Practice, Infusion Nurses Society (INS)
3. Strategies to Prevent Central-Line Associated Bloodstream Infections in Acute Care Hospitals, Society for Healthcare Epidemiology of America (SHEA)
4. National Patient Safety Goals, The Joint Commission
5. Clinical Practice Guidelines for Diagnosis and Management of Intravascular Catheter-Related Infection, Infectious Disease Society of America (IDSA)
6. Elimination Guide to Infections in Hemodialysis Settings, Association for Professionals in Infection Control and Epidemiology (APIC)
Where is Prevantics® Device Swab used?
Prevantics Device Swab is used on needleless access sites which include ports, hubs, connectors, dialysis connectors and blood collection applications. These needleless access sites are most commonly found on patients who require an IV (central line, mid-line, or peripheral line).
Why is the Prevantics® Device Swab solution beneficial?
By using an antiseptic to disinfect the needleless access site, you are decreasing the risk of the patient acquiring a bloodstream infection (BSI) which can be fatal to the patient. According to the CDC, “Disinfection of the devices with Chlorhexidine /alcohol solution appears to be most effective in reducing colonization”.
Some PDI products state "store at room temperature." What is the definition of room temperature?
For our EPA-regulated products, such as Sani-Cloth® and Sani-Prime™ brand products, room temperature is defined as an average temperature of 25◦ C (77◦ F) and within a temperature range of 15◦ C to 30◦ C (59◦ F to 86◦ F). For our FDA-regulated products, such as Prevantics® brand products, “controlled room temperature” indicates a temperature maintained thermostatically that encompasses the usual customary working environment of 20◦ C to 25◦ C (68◦ F to 77◦ F). SOURCE: USP 41-NF 36 General Notices and Requirements (August 1, 2013 First Supplements) Section 10.30.50. “Room Temperature” indicates the temperature prevailing in a working area. Section 10.30.60. Controlled Room Temperature
What is the shelf life for Prevantics® products?
The shelf life for Prevantics® Skin Antiseptics and Prevantics® Device Swab & Strip is 24 months or 2 years.
Product sizes and specifications are available in the convenient chart below.
| SKU Number | Product Size | Dimensions | Packaging | GTIN | Country of Manufacturer |
|---|---|---|---|---|---|
| C71200 | Large Swab | 1.59" x 2" | 10/150s | 20310819000035 | USA |
| B19600 | Swab | 1.59" x 1.31" | 10/100's | 20310819001520 | USA |
| B123ST | Strip | 1.59" x 1.31" | 10/160's | 20310819001773 | USA |