Interventional Care


We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
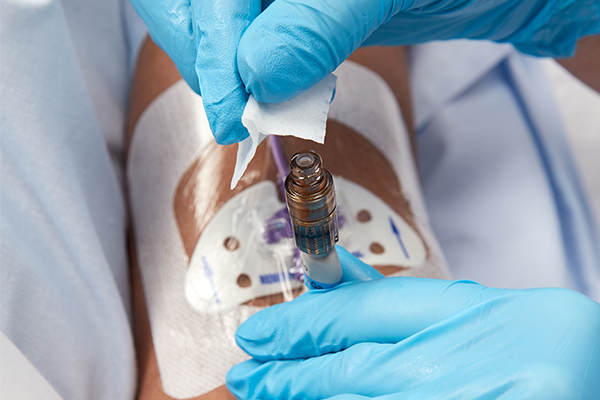
PDI Healthcare’s Prevantics® Device Swab was featured in a Product Spotlight in Surgical Products Magazine.
Surgical Products (surgicalproductsmag.com)
Excerpt:
The Prevantics Device Swab contains the first and only 3.15 percent (w/v) Chlorhexidine gluconate (CHG) and 70 percent (v/v) Isopropyl alcohol (IPA) solution to receive 510(k) clearance from the FDA for disinfecting needleless access sites prior to use. Featuring a 5 second scrub time followed by a 5 second dry time, Prevantics Device Swab helps improve staff compliance and reduces the risk of infection transmission. Available as a swab or in a strip format, the product is for use where and when healthcare professionals need it most, at the point-of-care. At the annual Scientific Meeting of the Association for Vascular Access, a Virginia-based healthcare facility presented a poster featuring the Prevantics Device Swab. After experiencing an increase in central line bloodstream infection (CLABSI) rates, the facility sought an effective way to improve disinfection compliance before every intravenous access. After Prevantics Device Swab was implemented hospital-wide in January 2016, CLABSIs decreased 76 percent year-over-year, from 17 documented infections in 2015 to four in 2016. The associated research concluded that the introduction of the Prevantics Device Swab increased compliance, provided fast and effective disinfection, decreased infections, and improved patient outcomes.