Interventional Care


We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?

In May 2021, AORN released the e-publication of their newly updated Guideline for Preoperative Patient Skin Antisepsis (with book publication sometime in 2022)1. Along with skin antisepsis, this update includes a recommendation for nasal decolonization, a comprehensive review of program implementation, and the recommendation for skin antisepsis. This is exciting news as the PDI Prevantics® skin products and Profend® Nasal Decolonization Kit fit nicely within the guideline recommendations to keep patients safe and reduce the risk of surgical site infection (SSI).
The primary objective of decolonization is to decrease the bacterial load of both the body and the nares.
Highlighting the risk our flora can play in developing an SSI, 30% of healthy adults have Staphylococcus aureus on their skin and in their nares2, and 80% of S. aureus SSIs can be attributed to the patient’s own flora3,4. It is important to note that S. aureus is a leading causative pathogen of SSI per the National Healthcare Safety Network (NHSN) reporting5 and nasal colonization with this organism increases the risk of SSI development up to 9 times2, 6-7. While mupirocin (a topical antibiotic) has historically been the gold standard for nasal decolonization, concerns with resistance and patient compliance have driven the science to support antiseptic efficacy. Povidone-iodine is now considered a viable option for successful nasal decolonization.
For many facilities, implementation of a nasal decolonization program is no small task! The guidelines outline the considerations for successful implementation (complete with supporting reference materials): from convening of an interdisciplinary team to assess patient/procedure risk to deciding on product type (antibiotic versus antiseptic), then to the selection of implementation strategy (universal, targeted, or blended), determination of protocol, and the selection of patient population and educational needs for sustainable use and compliance.
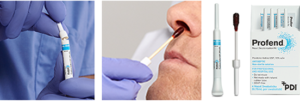
Profend® Nasal Decolonization Kit, a 10% povidone-iodine (PVP-I) product for nasal decolonization, fits nicely into the updated guideline recommendations to provide an effective and efficient patient safety intervention for the prevention of SSI. Profend® swabsticks are a broad-spectrum antiseptic providing gram-positive, gram-negative coverage and Candida sp. (antimicrobial) efficacy8. Following a 60 second application, Profend® antiseptic kills 99.7% of S. aureus at 10 minutes and 99.9% at 12 hours9– providing adequate/ample coverage during the patient’s most high-risk peri-operative period. Profend® Nasal Decolonization Kit fits into all strategy types mentioned, can quickly be applied with standing physician order or nurse-driven protocols, and efficiently fits into preoperative workflow. Profend® swabsticks will proactively defend patients from infection while also providing protection to patients with urgent surgical needs. Implementation of Profend Nasal Decolonization Kit includes robust education of staff, as well as materials for patient education, to drive compliance and sustainable use of this important intervention.
Along with meeting AORN guidelines for nasal decolonization, povidone-iodine is also supported by the Centers for Disease Control and Prevention (CDC) Strategies10 published in 2019 and provides anti-staphylococcal coverage per the Society for Healthcare Epidemiology of America/Infectious Disease Society of America (SHEA/IDSA) 201411 recommendation.
Nasal decolonization is not the sole intervention to prevent SSI; it is part of a larger group of interventions included in the new AORN guideline: preoperative bathing, surgical site hair removal (when indicated), and selection and application of surgical site antiseptic. Specifically, regarding skin antisepsis, the following recommendations around product selection are included:
PDI Prevantics® skin swabsticks are ideal for vascular access device (VAD) placement/ care/maintenance and minor surgical procedures. The Prevantics® Swabstick and Maxi Swabstick have a 7-day continued antimicrobial activity on the skin (aligning nicely with central line dressing change schedules and during the postoperative period), and pre-saturated swabsticks do not require cracking of a glass ampule. The dual-sided nature of the Swabstick’s applicator tip, in addition to a low profile with squared edges, makes it easy to navigate around an insertion site during VAD maintenance without fear of dislodging the catheter; the soft applicator tip is also gentle on the skin. The Prevantics® Maxi Swabstick provides ample solution to give antiseptic coverage to minor surgical procedures.