Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
“Human factors engineering is a discipline that studies the capabilities and limitations of humans and the design of devices and systems for improved performance. The principles of human factors engineering can be applied to infection prevention and control to study the interaction between the healthcare worker and the system that he or she is working with, including the use of devices, the built environment, and the demands and complexities of patient care”1.
Understanding the interactions among humans and other elements of a system can improve systematic performance, including the prevention of healthcare-associated infections. The three human factor engineering domains include a physical domain, a cognitive domain, and an organizational domain 2.
When thinking of a disinfectant wipe canister lid design, one may not immediately think of human factors engineering or that something as simple as a wipe dispensing lid can make such a difference in the day-to-day pace of a healthcare worker. When packaging is well designed, it fits seamlessly into the physical workflow of the healthcare worker, saving them time, and improving their efficiency, while also decreasing opportunities for errors and contamination that may lead to healthcare-associated infections3. As a nurse of 27 years, I can personally vouch for the fact that when things fit into your day so effortlessly that you use them constantly but do not “notice” them, they are truly designed well. Conversely, equipment, systems, and environmental factors that place barriers or time constraints on a nurse like me can cause untold frustrations, contributing to poor employee satisfaction, and ultimately poor patient outcomes.
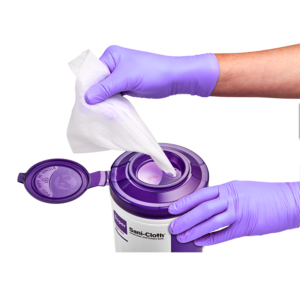
Disinfectant wipe canister lids have traditionally looked the same, with a small opening at the top that the wipe is threaded through. The wipes each have a perforated break line, which is supposed to allow for one wipe at a time to dispense. As an Infection Preventionist, part of my duties is auditing to ensure that employees utilize disinfectant wipes correctly. I have often seen a lid completely off at an angle and the wipes being taken out through the side of the canister, or that a lid top is left open. When I ask why responses are almost always the same – “it is too difficult to thread the wipes and keep them coming”, or “there is no convenient way to grab a large thread of wipes, except to take the lid off altogether”. Despite any inconvenience, it is important to note that not following the manufacturer’s instructions for use may cause a facility to receive citations during surveys.
So, the question is posed: If canister lids contribute to poor product compliance, why have canister lids not been redesigned knowing such flaws exist? The answer is simple! They now HAVE been redesigned!
This exciting news comes at a time when human factors engineering is an increased focus in infection prevention. PDI has incorporated insight gained from customer feedback and observation into a new lid, which addresses these issues. Designed with both a larger opening for access to more wipes, a smaller opening to select an individual wipe, and a snap-close lid, this innovation tackles the biggest frustrations for wipe users. Earl Adamy, Senior Director of Marketing, explains why PDI decided to develop the new lids, “Our goal is to provide our customers with a positive experience as they use our products. Too often, we observe customers struggle with canister lids, including loading the first wipe, dispensing multiple wipes, and leaving lids open. We felt that with some innovation, we could address these issues and improve the customer experience.”
I, like my colleagues at PDI, am thrilled and excited to deploy these canisters with the new lid design. If they fit into the flow of a workday and in some ways “become invisible,” they have genuinely been designed well. Taking human error out of the equation is a key to human factors engineering in packaging design. By making packaging easier for the user, less potential human error and frustration occur.
1.Critical Care Medicine 2010 Aug;38(8 Suppl):S269-81. Using human factors engineering to improve the effectiveness of infection prevention and control Judith Anderson, Laura Lin Gosbee, Mary Bessesen, Linda Williams. Veterans Affairs National Center for Patient Safety, Red Forest Consulting, LLC, Denver, CO, USA.
2.Yanke E, Carayon, and Safdar N. Translating evidence into practice using a systems engineering framework for infection prevention. ICHE 2014;35(9):1176-1182
3.Infect Control Hosp Epidemiol 2019 Jan;40(1):95-99. Infection prevention in long-term care: re-evaluating the system using a human factors engineering approach Morgan Jane Katz, Ayse P Gurses.