Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
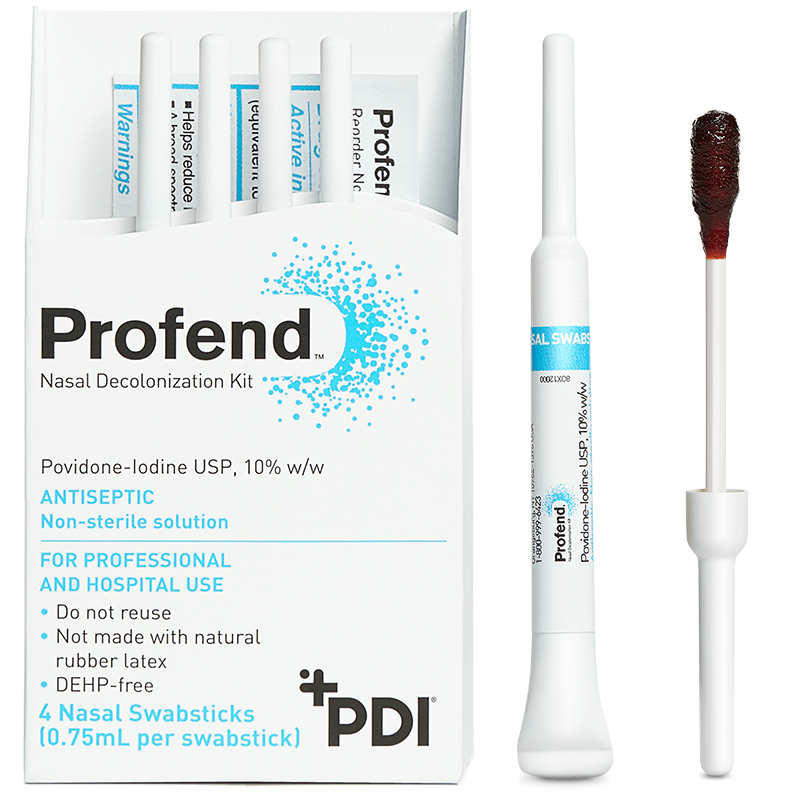

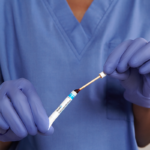
10% Povidone-Iodine (PVP-I) Nasal Swabsticks used to proactively defend your patients against infections related to S. aureus.
Profend® Nasal Decolonization Kit meets the new recommendations for nasal decolonization as per the AORN Guideline on Preoperative Patient Skin Antisepsis. Learn more.
Profend® Nasal Decolonization Kit meets nasal decolonization core strategy to prevent infections in high-risk surgeries, critical care and central IV catheter patients. Click here to learn more about the CDC Strategy for Nasal Decolonization.
Learn more about our Profend Nasal Decolonization Kit, the latest addition to the Interventional Care product portfolio targeting…
Povidone-iodine as an Alternative to Mupirocin for Nasal Decolonization: A New Domain for Antimicrobial Stewardship and Patient Safety…
This instructions for use sign contains step-by-step directions on how to properly open the applicator tube and for…
See how PDI Profend® Nasal Decolonization kits made a difference for infection prevention at Hunterdon Medical Center in…
Antimicrobial Efficacy of PDI Profend® Nasal Decolonization Kit 10% Povidone iodine vs. a Saline control against total bacteria…
PDI offers a broad range of evidence-based, market-leading Interventional Care, Environment of Care, and Patient Care solutions, all…
How is the Profend™ Nasal Decolonization Kit used?
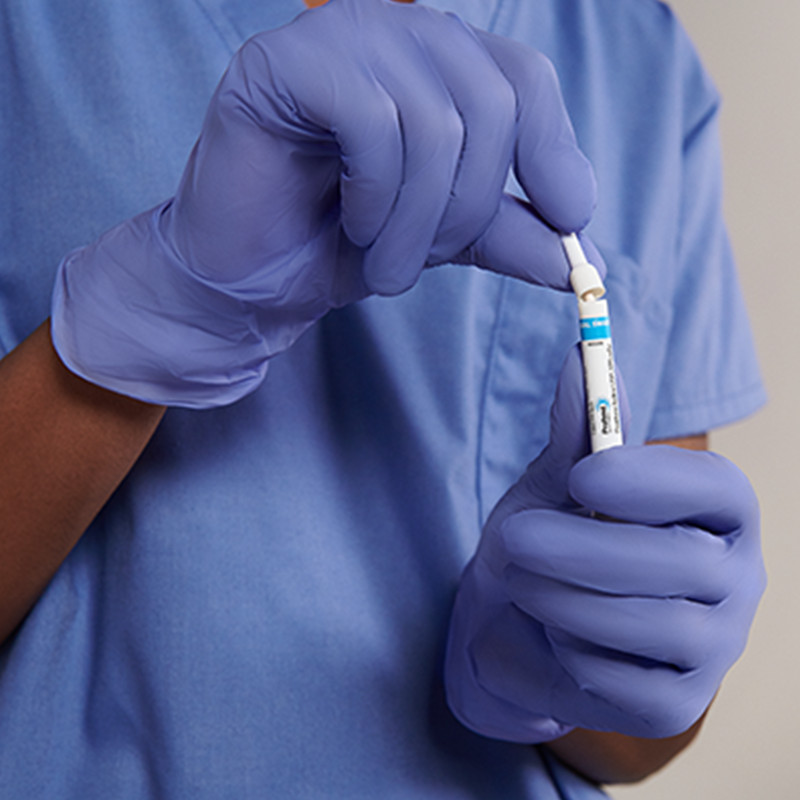
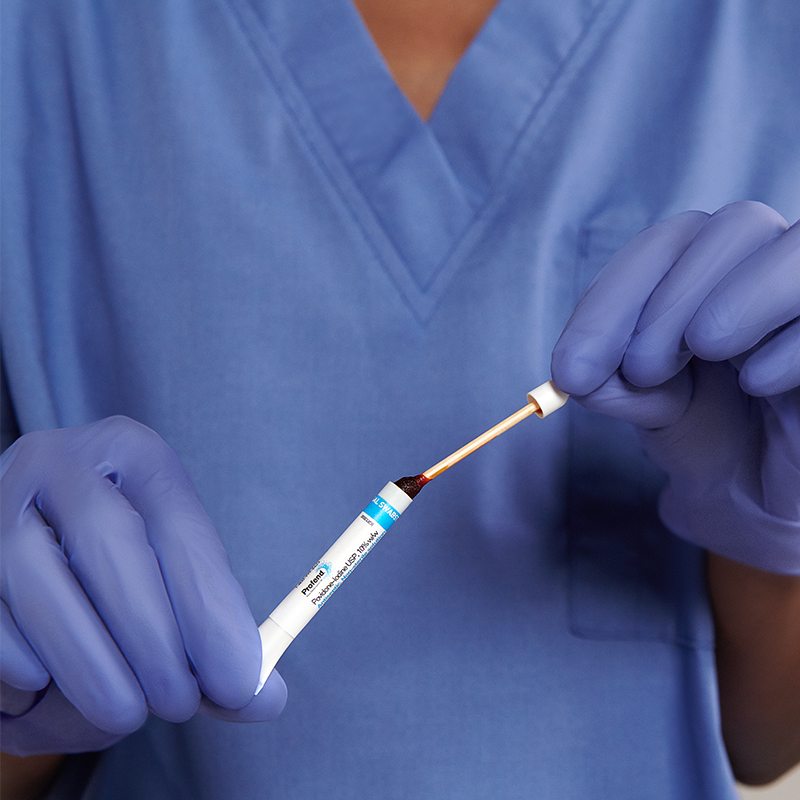
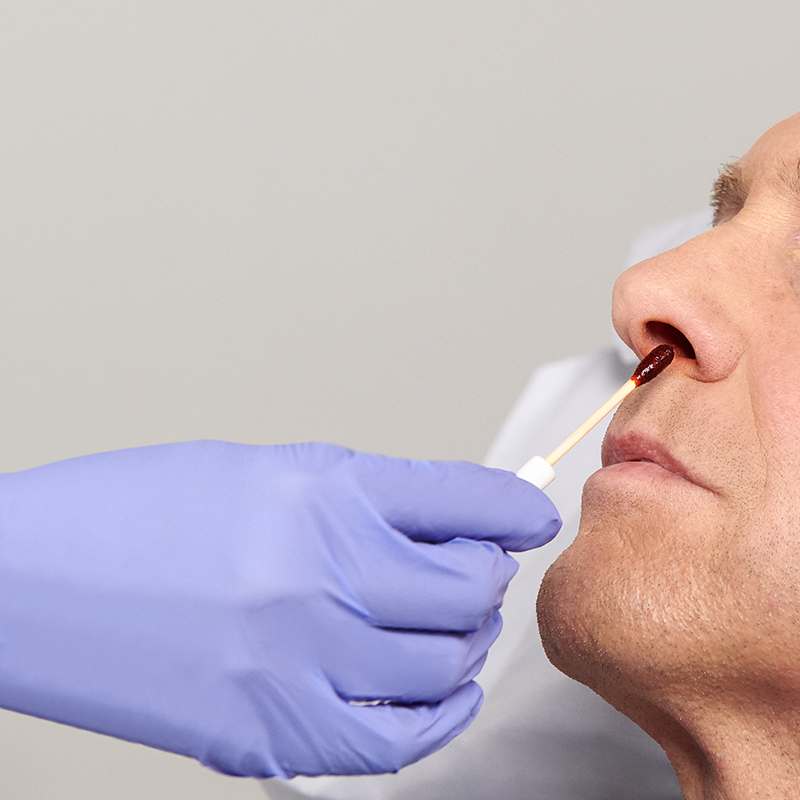
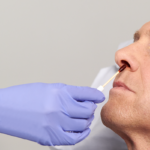
The Profend Nasal Decolonization Kit swabsticks are designed to be applied to the circumference of the patient nostril and anterior nares. Two swabsticks are applied to the nostril for 15 seconds per swabstick. Thus, using 4 swabsticks, total treatment time is 60 seconds.
What is the Profend™ Nasal Decolonization Kit?
The Profend Nasal Decolonization Kit is a pack of four swabsticks that are pre-saturated with 10% (w/w) Povidone-Iodine antiseptic solution and are applied to the nostrils/anterior nares of the nose to proactively defend patients against Staphylococcus aureus (S. aureus), Methicillin-resistant Staphylococcus aureus (MRSA) and other bacteria that can cause Surgical Site Infections (SSIs) and other healthcare-acquired infections (HAIs).
When is the Profend™ Nasal Decolonization Kit used?
The Profend Nasal Decolonization Kit can be used on patients who have tested positive for S. aureus and/or MRSA. As an alternative to a “test and treat” strategy, the ease and speed of application and economical design make Profend suitable for universal decolonization of all patients, saving time and money spent on patient testing. Regardless of decolonization strategy, Profend has proven efficacy—in a study of healthy volunteers, the Profend Nasal Decolonization Kit reduced S. aureus by 99.7% at 1 hour and 99.9%* at 12 hours post-application.With this in mind, for pre-operative use, clinicians may choose to apply the product to the patient 1-2 hours prior to surgery depending on hospital protocol.
SOURCE: PDI in vivo Study 0113-CTEVO.
Where is the Profend™ Nasal Decolonization Kit used?
The Profend Nasal Decolonization Kit can be used anywhere in a healthcare facility where there are patients who may be nasally colonized with S. aureus or MRSA and therefore have an elevated risk of developing an SSI or other HAIs.
Who uses the Profend® Nasal Decolonization Kit?
Perioperative nurses who care for patients prior to surgery and nursing staff in other areas of the hospital,ie. ICU, are the primary users of the Profend Nasal Decolonization Kit. Profend offers unique features valued by clinicians – a preference study showed that over 90% of nurses preferred Profend over other PVP-I nasal antiseptic products.
SOURCE: PDI user acceptance study.
Why is the Profend™ Nasal Decolonization Kit used?
Up to 30 percent of adults are nasally colonized with S. aureus and/or MRSA. S. aureus and MRSA account for over 30% of SSIs. Nasal colonization with these bacteria increases the patient risk of developing an SSI by 9 times. To help reduce this risk, Povidone-Iodine is used as it is a broad spectrum antiseptic that has been proven effective in reducing both S. aureus and MRSA. It offers an added advantage over the widely-used nasal antibiotic, mupirocin, in that there is no known bacterial resistance to it, thereby supporting antibiotic stewardship within the healthcare environment.
Some PDI products state "store at room temperature." What is the definition of room temperature?
For our EPA-regulated products, such as Sani-Cloth® and Sani-Prime™ brand products, room temperature is defined as an average temperature of 25◦ C (77◦ F) and within a temperature range of 15◦ C to 30◦ C (59◦ F to 86◦ F). For our FDA-regulated products, such as Prevantics® brand products, “controlled room temperature” indicates a temperature maintained thermostatically that encompasses the usual customary working environment of 20◦ C to 25◦ C (68◦ F to 77◦ F). SOURCE: USP 41-NF 36 General Notices and Requirements (August 1, 2013 First Supplements) Section 10.30.50. “Room Temperature” indicates the temperature prevailing in a working area. Section 10.30.60. Controlled Room Temperature
What important information can be found on the Master Label?
What is a Master Label?
The Master Label contains all of the approved uses for a given pesticide product and all associated labeling. Master Labels must be submitted for EPA approval. EPA-approved Master Labels are stamped “ACCEPTED” and placed in the official record. Labeling for a given product must not contain any text beyond that which is approved in the Master Label, except for supplemental labeling. The Master Label provides critical information about how to handle and use the pesticide product.