Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?




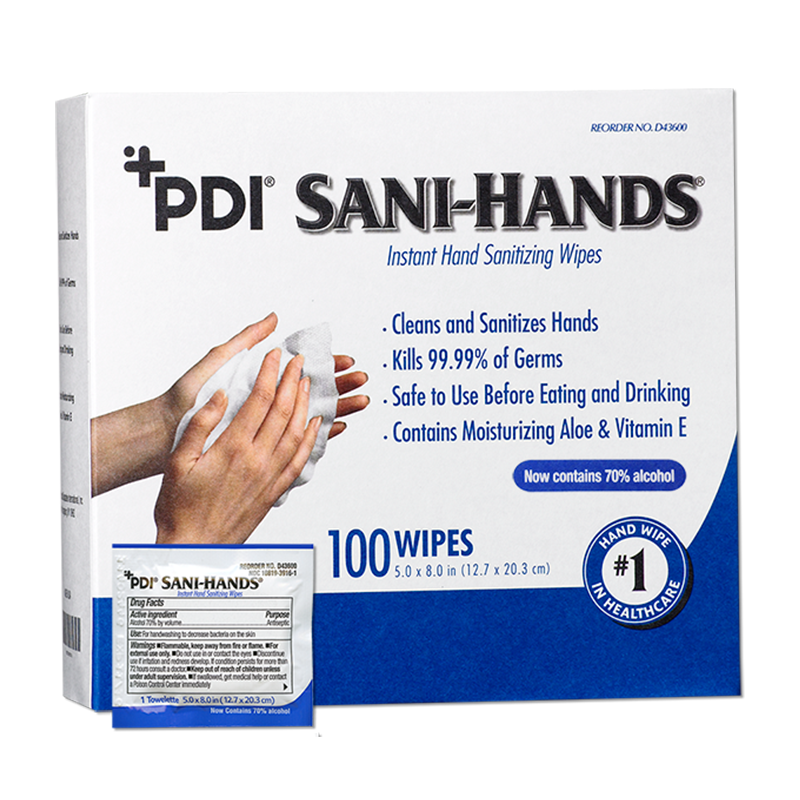





Sani-Hands® wipes is the #1 hand sanitizing wipe in healthcare, designed to support infection prevention and improve compliance at the point of care. Each pre-moistened wipe is formulated with 70% ethyl alcohol, meeting CDC and WHO guidelines for hand hygiene. Proven to kill 99.99% of many common bacteria and physically removes dirt and soil better than alcohol based gels or foams*.
Convenient and reliable, Sani-Hands wipes offer a practical hand hygiene solution for healthcare professionals, patients, residents, and visitors—anytime, anywhere. Ideal for patients with limited mobility who cannot get out of bed to clean their hands. The cost to treat an HAI can be as much as $58,500. By preventing just one, a patient hand hygiene program could pay for itself for an entire year.1
Sani-Hands is compliant with the SHEA/IDSA compendium for Hand Hygiene**, OSHA Bloodborne Pathogen Standard 29 CFR Part 1910.1030 (d)(2)(iv) and helps meet The Joint Commission 2018 National Patient Safety Goal #7.
SELECT A SIZE
15 seconds2
70% Ethyl Alcohol. Not made with natural rubber latex
Kills 99.99% of many common bacteria found on hands2
Wipe format removes dirt and soil better than gels and foams3,4
Safe to use before eating and drinking5
Contains soothing aloe & vitamin E
Designed to enable quick dispensing of wipes (large canister)
Manufactured in the US with domestic and foreign materials
This instructions for use sign can be placed around the facility to encourage proper hand hygiene technique with…
Small sign with icons to reinforce sanitizing hands before and after eating. Place on the bedside table to…
Did you know that our Sani-Hands® wipes help to remove bacteria more effectively than gels and foams from…
When running from patient to patient, it’s hard to always remember or find time to perform hand hygiene.…
Healthcare professionals work overtime to help save lives every day, and sometimes these jobs mean getting their hands…
Engage your patients in their care with this Sani-Hands video, which covers the importance of hand hygiene and…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
Patient hand hygiene is an important element of any infection prevention program. This “Patient Hand Hygiene” video series…
A downloadable listing of PDI’s point of care accessories for Sani-Cloth® and Sani-Hands® products, including 3-in-1 Wall Bracket®,…
Sani-Hands® Instant Hand Sanitizing Wipes are an ideal hand hygiene solution for staff, visitors, patients, or residents who…
PDI offers a broad range of evidence-based, market-leading Interventional Care, Environment of Care, and Patient Care solutions, all…
Do not let your guard down! As we enter respiratory virus season, it will be important to prepare for…
Are Sani-Hands® Instant Hand Sanitizing Wipes an FDA regulated product?
Sani-Hands Instant Hand Sanitizing Wipes are regulated by the US FDA as an OTC (Over-the-Counter) drug product covered under the Tentative Final Monograph for Healthcare Antiseptic Drug Products.
How long should I apply Sani-Hands® Instant Hand Sanitizing Wipes to the hands?
A contact time of 15 seconds provides a 99.99% reduction.
What is the expiration date on Sani-Hands® Instant Hand Sanitizing Wipes?
Sani-Hands wipes have a 24 month expiration date from the date of finished product manufacturing.
Some PDI products state "store at room temperature." What is the definition of room temperature?
For our EPA-regulated products, such as Sani-Cloth® AF3, Super Sani-Cloth, Sani-Cloth Bleach, Sani-Cloth Plus. Sani-Cloth Prime, Sani-HyPerCide® and Sani-HP1™ brand products, room temperature within a temperature range of 20◦ to 25◦ C (68◦ – 77◦ F) degree for room temperature and within a temperature range of 15◦ C to 30◦ C (59◦ F to 86◦ F). For our FDA-regulated products, such as Prevantics® brand products, “controlled room temperature” indicates a temperature maintained thermostatically that encompasses the usual customary working environment of 20◦ C to 25◦ C (68◦ F to 77◦ F).
SOURCE: USP 41-NF 36 General Notices and Requirements (August 1, 2013 First Supplements) Section 10.30.50. “Room Temperature” indicates the temperature prevailing in a working area. Section 10.30.60. Controlled Room Temperature
Product sizes and specifications are available in the convenient chart below.
| SKU Number | Product Size | Dimensions | Packaging | GTIN | Country of Manufacturer |
|---|---|---|---|---|---|
| P71520 | Bedside Pack | 8.4" x 5.5" | 48/20's | 20310819001087 | USA |
| P13472 | Medium Canister | 6" x 7.5" | 12/135's | 20310819000714 | USA |
| P15984 | Large Canister | 6" x 7.5" | 6/220's | 20310819000721 | USA |
| D43600 | Individual Packets | 5" x 8" | 10/100's | 20310819000745 | USA |
1.http://www.beckershospitalreview.com/finance/10-things-for-cfos-to-know-about-clabsis.html
2. Data on File (C0211)
3. Clinical data is representative of Sani-Hands (NDC #: 10819-50) 65.9% Ethyl Alcohol formulation vs. the Purell (NDC #: 21749 515) 62% Ethyl Alcohol formulation.
4. Data on file, PDI, Orangeburg, New York.
5. Food Code Recommendations of the United States Public Health Service Food and Drug Administration, 2017
*Compared to 70% Ethyl Alcohol (Foam) (and) (Gel) hand sanitizer products
“**Strategies to prevent healthcare associated infections through hand hygiene. Infection control and hospital epidemiology.vol.35 no.52. A compendium of strategies to prevent healthcare associated infections in acute care hospitals: 2014 updates (September 2014) pp. S155-S178.
https://www.jstor.org/stable/10.1086/677145#rid_rf116”